Resumen: La polilla del liquen Nyctochroa basiplaga (Erebidae: Arctiinae: Lithosiini) se encuentra desde México hasta Costa Rica. Al parecer ha sido catalogado, descrito e ilustrado con varios nombres diferentes desde su primer descubrimiento en el siglo XIX. Si todos estos se refieren a la misma polilla, puede haber hasta cinco nombres de especies diferentes asociados con la misma polilla. Aquí profundizo en la compleja historia de esta polilla. Se necesita una revisión taxonómica formal de todos estos taxones.
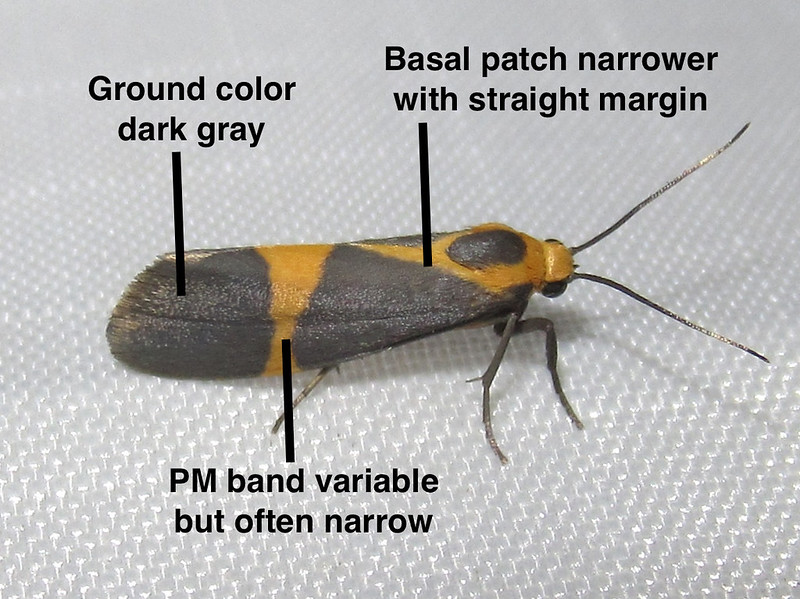
Take a close look at this image of a lichen moth from Oaxaca, Mexico, photographed by @juanmaguey in October 2019:

(full-size image:) https://inaturalist-open-data.s3.amazonaws.com/photos/181343415/original.jpeg
and then read on, if you dare:
In the course of researching the lichen moth genus Eudesmia, I got sidetracked for the past few days looking up old descriptions of some of the unknown and unrecognized lichen moth species (Erebidae: Arctiinae: Lithosiini) in various checklists. The lists on the FUNET site are particularly useful (Thank You, Markku Savela! See links at bottom of post). Not uncommonly, species described by authors in the 19th Century have bounced around in various genera over the succeeding 150 or so years, and lepidopterists of that era frequently named new species without a comprehensive review of previous taxa, creating numerous synonyms which needed to be sorted out later by subsequent generations of researchers. Such seems to be the case of a lichen moth originally named Nyctochroa basiplaga by Felder & Felder in 1874. That’s the earliest name I can find for a moth that seems to have been named and described anew over and over again. In their massive volumes documenting the “Voyage of the Frigate Novara”, Felder & Felder illustrated this species from Mexico (Vol. 2, pl. 106, fig. 27) and listed it in an index but did not describe it.
![Nyctochroa basiplaga Felder 1868 [1874] Voyage pl 106 f 27 copy](https://live.staticflickr.com/65535/53417444212_eb819057a8_w.jpg)
https://archive.org/details/umdieerdeindenja03akad/page/n262/mode/1up?view=theater
Spoiler alert: I think ALL of the following names refer to this same moth which ranges from Mexico to Costa Rica. These are listed alphabetically, then in chronologic order by date of publication. The current generic placement of each species epithet is noted in bold font.
abdulla Dyar, 1917 [genus Cisthene]
abdulla, Draudt, 1925 [genus Cisthene]
abdulla FUNET [genus Eucyclopera] (accessed 22 Dec 2023)
basiplaga Felder, 1874 [genus Nyctochroa; same on FUNET]
basiplaga, Druce, 1881 [genus Nyctochroa]
basiplaga, Hampson, 1920 [genus Cyanarctia]
carpintera Schaus, 1910 [genus Brycea]
carpintera Hampson, 1920 [genus Cyanarctia]
carpintera FUNET [genus Eucyclopera] (accessed 22 Dec 2023)
ira Druce, 1889 [genus Ptychoglene; also Druce in BCA]
ira Hampson, 1900 [genus Euryptidia]
ira Draudt, 1919 [genus Euryptidia]
ira Scott Chialvo et al, 2018 [genus Euryptidia]
ira NHM(UK) [genus Paratype; listed on FUNET] (accessed 22 Dec 2023)
lithosiaphila Dyar, 1910 [genus Hypomolis]
In 1889, Herbert Druce described a moth from Mexico essentially identical to that of the Felders' as Ptychoglene ira (Ann. Mag. Nat. Hist. (6)4:90), calling it a “very distinct species”, and illustrating it in the Biologia Centrali-Americana (BCA Lep-Het 2:398; 3: pl. 78, fig. 6).

https://www.biodiversitylibrary.org/page/19301582
https://www.biodiversitylibrary.org/page/591374
https://www.biodiversitylibrary.org/page/591678
Curiously, also in the BCA (Lep-Het, I, p. 155, 1881), Druce had dutifully listed the Felders' Nyctochroa basiplaga but noted that it was “unknown to me.” In his 1900 Catalogue of the moths in the British Museum, Hampson moved ira into the genus Euryptidia, probably based on wing venation characters.
Perhaps unaware of the Felder volumes or the BCA (?), Harrison Dyar published a new species from Mexico in 1910, Hypomolis lithosiaphila (Proc. USNM 38:235, published on June 7, 1910). His description, although typically brief, is quite similar to the moths illustrated by Felder and Druce.
https://archive.org/details/proceedingsofuni381911unit/page/235/mode/1up?view=theater
Complicating matters, just four months later in October 1910, unaware of Felder’s, Druce’s, or Dyar’s species, William Schaus described a “new” lichen moth Brycea carpintera (Ann. Mag. Nat. Hist. (8) 6 (34):406) from Costa Rica which is apparently identical to the earlier species.
https://archive.org/details/annalsmagazineof861910lond/page/406/mode/1up?view=theater
This is a classic case of researchers in that era working at cross-purposes or quite ignorant of each others' efforts. [Historical sidenote: Schaus, a native New Yorker, spent much time in London as well as traveling in Central and South America collecting Lepidoptera. He later became associated with the U.S. National Museum in about 1919, working alongside Harrison Dyar. Their interesting and sometimes contentious relationship is chronicled by Marc Epstein in his biography of Dyar, "Moths, Myths, and Mosquitos", Oxford University Press, 2016.]
In 1917, Dyar named yet another lichen moth from Mexico, this time placing it in the genus Cisthene, as Cisthene abdulla (Insec. Inscit. Mens. 5(1-3):10), but once again the description is essentially identical to Felder’s basiplaga, Druce’s ira, Dyar’s own lithosiaphila, and Schaus’s carpintera. Dyar's placement of his new species in "Cisthene" followed Hampson's concept of the latter genus which now mainly refers to Eudesmia lichen moths (sensu lato; see Sexton 2022). Neither genus represents a satisfactory placement of abdulla (see Draudt's and Seitz's notes, below).
https://archive.org/details/insecutorinsciti56191718wash/page/n17/mode/1up?view=theater
So by 1920 there were five names in five different genera for essentially the same type of lichen moth. The descriptions were almost identical word for word, and Felder’s and Druce’s images were similar enough to suggest that they were probably the same species, unless there was some interesting mimicry complex in play!
In his 1920 supplement to the Catalogue of Leps in the British Museum, Hampson rearranged some of these species at the genus level, placing them in the genus Cyanarctia, carrying C. carpintera forward as a valid species, but lumping Dyar’s lithosiaphila under “Cyanarctia" basiplaga (Cat. Lep. Phal. Br. Mus. Suppl. 2:328-329). Hampson did not address Dyar’s Cisthene abdulla, probably because the British Museum had no specimens at hand. In moving Felder’s basiplaga to the genus Cyanarctia and lumping Dyar’s lithosiaphila with it, he confessed, like Druce, that the species was “unknown to me”, As a fallback choice, Hampson illustrated only the USNM type specimen of Dyar’s lithosiaphila. His illustration looks veeery familiar!

https://archive.org/details/catalogueoflepid12brit/page/n255/mode/2up?view=theater
Draudt (in Seitz, "1913", Macrolepidoptera of the World, vol. 6, p. 467, and pl. 39 row l; published in a 1925 supplement entitled, "Additions: Lithosiinae") listed Cisthene abdulla Dyar, and added a note that "this insect [probably] coincides with Cyanarctia basiplaga = Hypomolis lithosiaphila." Later in that same "Additions" supplement, Seitz himself went on to (apparently) synonymize Cyanarctia basiplaga (Felder), lithosiaphila Dyar, Cisthene abdulla Dyar, and Cyanarctia carpintera (Schaus). This seems to leave only Ptychoglene/Euryptidia/Paratype ira out of the fold.

This species—or this set of names—has not been addressed in detail in nearly a century. There has been a lot of recent research on the biochemistry and phylogenetics of Tiger and Lichen Moths, and even a comprehensive catalogue of a portion of the Neotropical Arctiinae, but none of these efforts have addressed the present set of named species in any useful manner. Scott Chialvo et al. (2018; in the online journal Molecular Phylogenetics and Evolution) included Euryptidia ira in their molecular studies but did not include any of the other “species” mentioned above and came to no conclusions on the proper placement of ira in the grand scheme of things. To my knowledge, @juanmaguey's image (above) is the First Photograph of a Living Specimen of whatever species it is, although there are piles and piles of unidentified Tiger and Lichen Moths in Mexico and Central America. I wandered through them earlier today and did not find any other obvious candidates for this taxon.
So I’m just proposing that Seitz in his 1925 "Additions" was mostly correct, namely, that the several names, in several genera as listed above, all represent the same lichen moth. It will fall to researchers with access to museum specimens and all the modern tools of taxonomy to sort this all out and confirm or refute my guess.
Here are links to Markku Savela’s pages on FUNET for these various species, in the genera where they are currently listed:
https://ftp.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/noctuoidea/arctiidae/lithosiinae/eucyclopera/
https://ftp.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/noctuoidea/arctiidae/lithosiinae/nyctochroa/
https://ftp.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/noctuoidea/arctiidae/lithosiinae/paratype/
























![Nyctochroa basiplaga Felder 1868 [1874] Voyage pl 106 f 27 copy](https://live.staticflickr.com/65535/53417444212_eb819057a8_w.jpg)